XLA is a rare genetic primary immunodeficiency caused by a mutation in a gene named BTK. This genetic mutation leads to a failure of B-cell maturation and disrupts the ability of the patients to produce antibodies. Without treatment, XLA patients would suffer from recurrent infections that will eventually lead to death.

Current therapy consists of life-long administration of pooled human antibody replacement therapy and targeted antimicrobial agents. This treatment is insufficient, and even with treatment, XLA patients have a reduced lifespan and suffer frequent and serious health complications.
BTK-001 is a lentiviral vector-based gene therapy designed to re-engineer XLA patient’s own stem cells by delivering the BTK gene directly to stem cells. Upon stem-cell reprograming, patients regain the ability to develop B-cells and produce antibodies, essentially being cured from the disease after a single treatment.
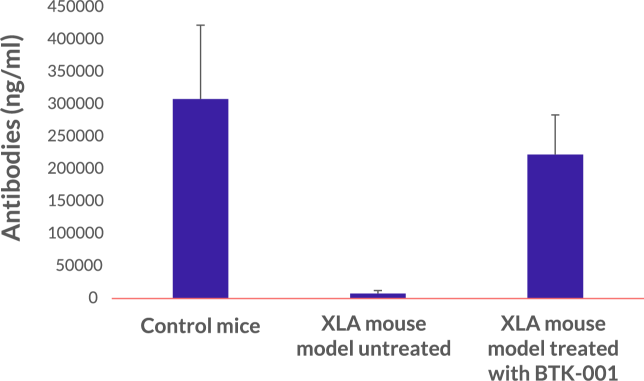
BTK-001 treatment restores the capacity of mice with XLA to produce antibodies





Sefi is a 22-year entrepreneur who founded a Nasdaq-listed company. He has a proven track record of assembling exceptional teams and building technology ahead of its time.
Sefi founded HUB Cyber Security, which reached a peak market capitalization of $350 million. He also advised Bancor, which raised $153 million and reached a peak market capitalization of $800 million.
Currently Sefi is heading the investment team in a privately owned family office.
Sefi holds a B.Sc. in Business Administration and Economics from the University of Tel Aviv.




Mr. Adam Brian heads Lonza Personalized Medicine, which focuses on developing and bringing to market tools that enable research and development, scale out, and commercialization of patient scale cell therapies, with an emphasis on decentralized and point of care manufacturing. Mr. Brian is a results-oriented leader with extensive experience in the life science and medical device industries. Proven track record of increasing revenue, improving operations, and building and leading high-performing teams.

Dr. Eytan Abraham heads Resilience cell, gene and nucleic acids Franchises. Dr. Abraham holds a Ph.D. in developmental and molecular biology from the University of Maryland Biotechnology Institute, and a post-doctorate in cell-therapy and tissue engineering from the Harvard-MIT Biomedical Engineering Center and Harvard Medical School. Dr. Abraham is an experienced scientist and business leader with expertise in basic and applied biological and cell therapy R&D as well as in management, technology, and commercialization.

Anat Naschitz is a lifescience investor and entrepreneur, with 27 years of experience across biotech, pharma, digital health and medical devices.
Anat co-founded and co-led OrbiMed Israel, an Israel focused VC fund and part of the leading, $20bn global healthcare investment firm, and was previously with Apax, the €60bn private equity firm, where she started her investment career. Previously Anat was an Associate Partner with McKinsey in London, where she advised the world’s preeminent pharmaceutical companies on strategy, acquisitions and spinouts.
Throughout her career Anat has founded companies and nurtured them through success. Examples include 89bio (Nasdaq:ETNB), developing a likely best in class therapeutic for NASH, which she co-founded and spun out of Teva, taking it public on Nasdaq 18 months post creation, currently trading at $1bn; Sobi, which evolved out of a Pharmacia spinout and currently trading at $7.4bn; and many others.
Currently, Anat is co-founder and CEO of 9xchange, a biopharma marketplace, creating transparency and enabling the ecosystem to monetize pipelines and discover assets whose availability was unknown, through a seamless, initially anonymous, AI-driven process.
Anat earned her MBA at Insead in France and her LLB at Tel Aviv University.

Dr. Yeal Weiss is currently CEO of Mahzi Therapeutics, a company focused on the development of therapies for ultra-rare genetic neurodevelopmental disorders. Dr. Weiss completed her MD at Hadassah Medical School at the Hebrew University in Jerusalem and her PhD at the Weizmann Institute of Science in Rehovot, Israel. She has over 20 years of industry experience in medical/clinical and business development roles at Genzyme, Merck and Ultragenyx. Dr. Weiss is a member of the NIH driven Bespoke Gene Therapy (BCTG) consortium, ASGCT translational committee, N=1 collaborative and is a 2022 Termeer Fellow. Board member/advisor to ADNP and FOXG1 foundations.

Noam is a physician with 20 years of experience in the field of complementary medicine (Brighton University). He formerly headed the Chinese Medicine Unit at Sheba Hospital. Noam is the founder and CEO of the Association for Noga (Her Way), which develops advanced therapies for ultra-rare diseases.


Liron brings many years of experience in cell- and gene-therapy translation. Prior to joining NOGA Therapeutics, Liron headed the discovery and the early-phase development of oncology at Enlivex Therapeutics. Prior to that, Liron led the hemophilia A program (currently in phase III) at Spark Therapeutics, USA. Liron holds a PhD from and conducted postdoctoral research at the Technion.


Noam previously co-founded Emendo Biotherapeutics, a leading CRISPR company that was acquired by Anges, Japan, in 2020, for $295M. As an expert on intellectual property strategies at Ingenium IP, Noam advised dozens of biomed companies on building their IP-business strategy. Noam holds a PhD from the Weizmann Institute of Science in the field of DNA damage repair.
